When organic material is added to soil three general reactions take place. 3-5 days after death the body starts to bloat and blood-containing foam leaks from the mouth and nose.

Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
2 Describe what happens in each of the five main types of chemical reactions.

. A decomposition reaction is the opposite of a synthesis reaction. Several weeks after death nails and teeth fall out. Car airbags are a very good example of a decomposition reaction in action.
A compound breaks down into separate elements and compounds Rank the following in order of increasing molar mass. The process may occur in a. 3 Balance the equations and identify the type of each reaction as.
Combustion is an exothermic reaction in the form of heat and light that releases energy. The bulk of the material undergoes enzymatic oxidation with CO2 water energy and heat as the major products. These re actions often involve an energy source such as heat light or electricity that breaks apart the bonds of compounds.
The combustion of these results in the decomposition of the fuel and the evolution of a volatile gas. Very slowly decomposed. Simple decomposition products Aerobic CO 2 H 2 O NO 2 SO 4.
Give one example of a chemical reaction from each category. Which statement describes what happens during a decomposition reaction. Time Consider the reaction 2NaN3 2Na 3N2 which is the first reaction that occurs when an air bag expands in a car undergoing a collision.
A decomposition reaction is a type of chemical. Perform a Double Displacement Reaction Lab Results Describe what happens when solutions of NaOH and NiCl2 are combined. Usually energy is required to.
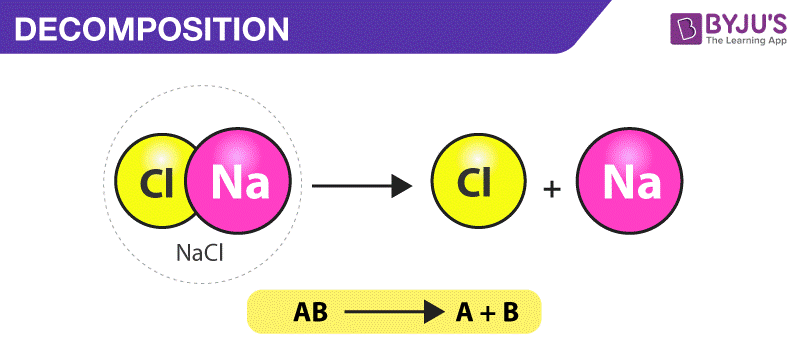
A decomposition reaction is a type of chemical reaction in which one reactant yields two or more products. These reactions often involve an input of energy in the form of heat light or electricity to break down the compounds. There are many types of chemical reactions.
In other words it can also be considered as a reaction in which reactants on providing activation energy breaks down decomposed to products. This reaction can also be known as the opposite of a composition reaction. H2O2 I2 LiF CH6N.
24-72 hours after death the internal organs decompose. What is Decomposition Reaction In a reaction in which one compound breaks down into two or more simpler compounds. The reaction will shift to the left toward the reactants.
The reaction will culminate in the mixture violently shooting upwards out of the flask. Ni2 and Cl- What ions are present in an aqueous solution of NaOH. _____g _____g _____g.
AnswerNa and OH Conclusions Write a balanced equation for the. A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more elements or new compounds. AB A B In some cases the reactant breaks into its component elements but a decomposition may involve breakdown into any smaller molecules.
This reaction happens very quickly as you might suspect. In the process of the decomposition reaction many complex compounds break down into multiple simpler compounds. These reactions are often classified by what they form or what happens during the course of the reaction.
Changes in which measurement do not shift the equilibrium of a chemical system. Decomposition reactions are a type of chemical reaction that involves breaking down a compound into smaller compounds or individual elements. The opposite of this type of reaction is a synthesis in which simpler reactants combine to form a more complex product.
The rate at which oxygen diffuses to the surface determines how quickly the solid carbonaceous residue burns. Thermal decomposition is a chemical reaction that happens when a compound breaks down when heated. Get 1-on-1 help from an expert tutor now.
The chemical bonds between the atoms of the decomposing compound are broken and then rearranged between the atoms in new ways to make the products. Decomposition reactions are also known as analysis reactions or chemical breakdowns. In a decomposition reaction a single compound breaks apart into two or more elements or compounds.
38Describe the reactions of copper and magnesium with water Dont react immediately you probably wont see any reaction Metals and oxygen 39Describe what happens when copper reacts with oxygen Outside becomes black this is copper oxide 40Describe what happens when magnesium reacts with oxygen Bright spark. Fats waxes resins lignins etc. Google Still stuck.
Thermal decomposition reactions happen at high. The separation of a substance or material into two or more substances or materials that might differ from each other and from the original or unique substance are called as the decomposition reaction. Decomposing is the process of breaking down.
Combustion is a chemical reaction between a fuel and an oxidant usually atmospheric oxygen high-temperature exothermic heat releasing redox oxygen adding which creates oxidized often gaseous products in a mixture called smoke. Combination decomposition single replacement double replacement or combustion. Decomposition reaction releases heat energy making it an exothermic and fizzy reaction As the decomposition of hydrogen peroxide continues a lot of pressure will quickly build up in the flask due to the volume of oxygen gas being produced.
____HgO s ____Hg l ____O2 g Molecular weight. A surface temperature in the range of 4000 C to 8000 C is required for this combustion to occur. Balance the following decomposition reactions each time ensuring that the law of conservation of mass applies.
Up to 24 cash back Describe what happens in a decomposition reaction. 8-10 days after death the body turns from green to red as the blood decomposes and the organs in the abdomen accumulate gas. Balance the following equations and label them as either a synthesis or.
In a decomposition reaction a compound is broken into smaller chemical species.
Decomposition Reaction Definition Types Examples Uses

0 Comments